Experiment Name: Scouring and bleaching of 100% cotton knitted fabric at combined stage.
Theory: Scouring is the process
of removing the impurities such as oil, fat, wax dust and dirt from the
textile material to make it hydrophilic. Bleaching is the chemical
treatment for removal of natural coloring matter from the fabric. The
source of natural color is organic compounds with conjugated double
bonds, by doing chemical bleaching the discoloration takes place by
breaking chromophore. The material appears whiter after the bleaching.
Nature of Sample : 100% cotton knitted gray fabric.
Apparatus Required :
1. Beakers.
2. Glass Rod.
3. Pipette.
4. Measuring Cylinder.
5. pH meter.
6. Tri-pod Stand.
7. gas burner.
8. Thermometer.'
9. Pot.
Process Sequence:
Collection of 100% cotton fabric
////
Scouring and bleaching at 100deg C
////
cold rinsing
////
hot wash
////
cold rinsing
////
drying
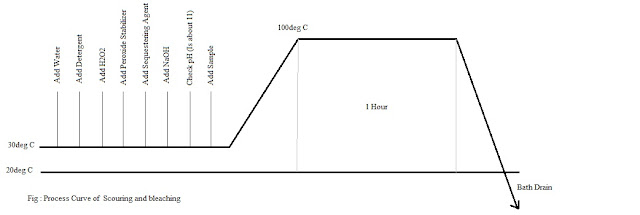
Recipe of Scouring and bleaching:
Detergent: 1 g/L (Stock Solution - 1%)
NaOH: 3 g/L (Stock Solution - 2%)
H2O2 : 4 g/L (Stock Solution - 3%)
Peroxide stabilizer : 1 g/L (Stock Solution - 1%)
Sequestering agent: 1g/L (Stock Solution - 1%)
Temperature:100@C
Time: 1 Hour
Fabric Weight: 5gm
M:L = 1:30
Calculation:
Total Liquor required
Fabric Weight = 5gm
M: L = 1:30
Required amount of liquor = 5 X 30
= 150mL
Detergent : 1g/L
= (150 X 1g/L) / (1% x 1000)
= 15ml
NaOH : 3g/L
= (150 X 3g/L) / (2% x 1000)
= 22.5ml
H2O2 : 4g/L
= (150 X 4g/L) / (3% x 1000)
= 20ml
Peroxide stabilizer : 1g/L
= (150 X 1g/L) / (1% x 1000)
= 15ml
Sequestering agent : 1g/L
= (150 X 1g/L) / (1% x 1000)
= 15ml
Initial Water required = Total liquor - Chemicals
= 150 - (15+22.5+20+15+15)
= 150-87.5 mL
= 62.5mL
Function of Chemicals:
Detergents:
Emulsify fats, oils and waxes, and suspend other dust dirt etc.
NaoH: Neutralizes acidic medium. maintain alkaline medium for perfect operation.
Sequestering agent:
Deactivate metal ions.
Peroxide Stabilizer: The decomposition of peroxide under the influence of alkali is adjusted by stabilizer.
H2O2: It is the bleaching agent. It used to produce permanent whiteness on fabric.
Process Curve
Sample Attachment and Observation:
Comments: The treated fabric is whiter compare to the non treated fabric.
Pre-cautions:
- All chemicals measure are taken properly.
- Temperature maintain properly.
- Burner used very carefully.
- Time maintain according to recipe.
Conclusion: ....................................